The Jackson Laboratory (JAX) has over 80 years of expertise in metabolic research. We maintain the world's largest collection of clinically relevant mouse models for obesity, diabetes, and other metabolic disorders. Our standard and customized in vivo preclinical research services support a range of research needs from disease mechanism studies to therapeutic target validation and efficacy testing. From early-stage therapies to novel biological insights, JAX's models, research services, and expertise can help you generate translational data to drive discovery and therapeutic advancement.
With our advanced phenotyping capabilities and deep scientific expertise, JAX empowers researchers to advance metabolic research and breakthroughs in diabetes, obesity, and related disorders.
Our Expertise
JAX provides comprehensive in vivo metabolic phenotyping services to support preclinical drug discovery and translational research.
Chronic and acute dosing studies to assess drug efficacy in mouse models of metabolic disease, with clearly defined control groups and assessments of side effects.
Examination of pancreatic tissue through histological analysis (H&E), including:
Comprehensive body composition analysis, including:
Assessment of:
Metabolic assessment through indirect calorimetry, including:
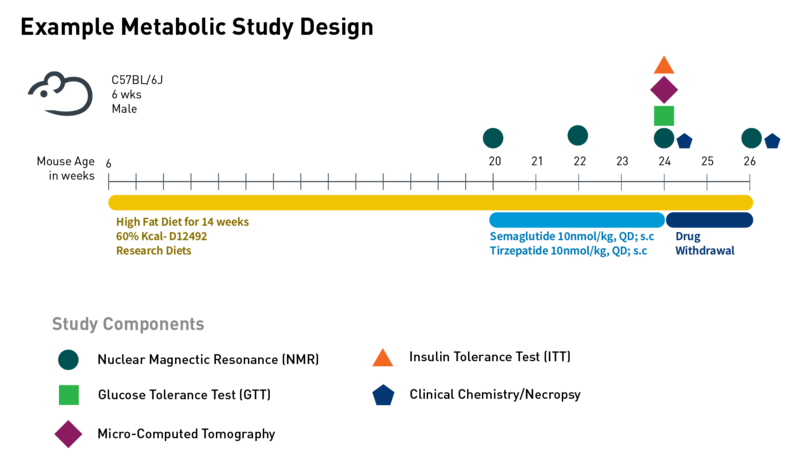
JAX Preclinical Services are managed by experienced study directors and staff dedicated to helping you obtain the critical data needed for your research. See the sample study below—custom studies are also available. For more details and additional examples, click to see more Metabolic research data.
Body weight trajectories during treatment and post-withdrawal. Vehicle-treated mice maintained stable weight, while Semaglutide and Tirzepatide reduced body weight by ~7% and ~30%, respectively. Upon withdrawal, Semaglutide and Tirzepatide groups regained ~5% and ~20% of body weight.
Fat content after 4 weeks of treatment. Semaglutide reduced fat mass by 16%, while Tirzepatide induced a 62% reduction.
In vivo 3D microCT imaging of adipose and lean tissue (Quantum GX, PerkinElmer) after 4 weeks of treatment. Adipose depots were segmented based on density and outlined manually using Image J. Subcutaneous fat is shown in green, visceral fat in red, and lean mass in blue. Both treatments reduced subcutaneous and visceral adiposity. n = 8-9 per group.
Lean mass changes (grams). Lean mass increased by 2.5% in Vehicle controls, but declined by 1% and 7.8% with Semaglutide and Tirzepatide, respectively.
With so many models available, choosing the most appropriate mouse model for your metabolic study can be challenging. During this webinar, we discuss the strengths and limitations of popular mouse models of human type 2 diabetes, obesity, and metabolic dysfunction-associated steatohepatitis (MASH).
Not what you're looking for?
Visit our Resource Hub